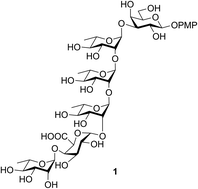
Chemical synthesis of the hexasaccharide related to the repeating unit of the capsular polysaccharide from carbapenem resistant Klebsiella pneumoniae 2796 and 3264†
Abstract
The total synthesis of the hexasaccharide repeating unit of the CPS from carbapenem resistant K. pneumoniae 2796 and 3264 is reported using a sequential glycosylations approach. The total synthesis has been accomplished by glycosylation of rationally protected monosaccharide synthons derived from the commercially available sugars. The required uronic acid on the galactose moiety was successfully installed by a TEMPO-mediated late stage oxidation. The glycosylations were performed by the NIS-mediated activation of thioglycosides using H2SO4-silica as the promoter. Chloroacetate group was extensively used as a temporary protecting group to facilitate stereoselective glycosylations.

 Please wait while we load your content...
Please wait while we load your content...