Synthesis, structural and electrochemical study of O3–NaNi0.4Mn0.4Co0.2O2 as a cathode material for Na-ion batteries
Abstract
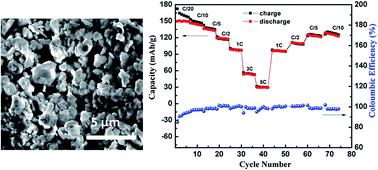
Hexagonal layered O3–NaNi0.4Mn0.4Co0.2O2 is prepared by a mixed hydroxide solid state reaction method at an optimum temperature of 800 °C. The Rietveld refined PXRD pattern reveals single phase formation with space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m. Galvanostatic electrochemical studies reveal reversible sodium de-insertion/insertion with concomitant structural phase transitions. In the voltage window of 2.2–3.8 V, the first discharge capacities are 150 and 135 mA h g−1 at C/20 and C/6.5 current rates, respectively. The capacity retention is 80% after 33 discharge cycles at C/20 rate and 78% after 40 discharge cycles at C/6.5 rate. When the voltage window is increased to 1.6–4.5 V, the initial discharge capacity is 173 mA h g−1 at C/10 rate and a capacity retention of 63% is observed after 10 discharge cycles. The O3 phase undergoes a series of structural transformations from O3, O′3, P3, P′3 and P3′′ during charging and reverts to the O3 phase upon insertion of Na during discharge. Structural stability is evident from ex-situ XRD studies even after 33 cycles, when cycled in the voltage window of 2.2–3.8 V at C/20 rate.
m. Galvanostatic electrochemical studies reveal reversible sodium de-insertion/insertion with concomitant structural phase transitions. In the voltage window of 2.2–3.8 V, the first discharge capacities are 150 and 135 mA h g−1 at C/20 and C/6.5 current rates, respectively. The capacity retention is 80% after 33 discharge cycles at C/20 rate and 78% after 40 discharge cycles at C/6.5 rate. When the voltage window is increased to 1.6–4.5 V, the initial discharge capacity is 173 mA h g−1 at C/10 rate and a capacity retention of 63% is observed after 10 discharge cycles. The O3 phase undergoes a series of structural transformations from O3, O′3, P3, P′3 and P3′′ during charging and reverts to the O3 phase upon insertion of Na during discharge. Structural stability is evident from ex-situ XRD studies even after 33 cycles, when cycled in the voltage window of 2.2–3.8 V at C/20 rate.

 Please wait while we load your content...
Please wait while we load your content...