Porous ceramic stabilized phase change materials for thermal energy storage
Abstract
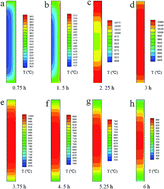
This paper aimed to develop a novel form-stable composite phase change material (PCM) by infiltrating molten Na2SO4 into a mullite-corundum porous ceramic preform (M-PCP). Sufficient coal-series kaolinite (Kc), aluminum hydroxide, aluminum fluoride and graphite were mixed and subsequently heated in air at 1450 °C to produce M-PCP. The microstructure, thermal properties and thermal reliability of the composite PCMs were characterized by thermogravimetric and differential scanning calorimetry, X-ray diffraction and scanning electron microscopy. The results indicate that the M-PCP/Na2SO4 was 54.33 J g−1 at its melting temperature of 882.17 °C. Impregnation experiments and numerical simulation demonstrated high-temperature chemical compatibility and wettability between molten Na2SO4 and M-PCP. The M-PCP/Na2SO4 composite showed good thermal stability after 30 thermal shock cycles, and could potentially be used in the thermal energy storage field.

 Please wait while we load your content...
Please wait while we load your content...