The dynamics of the β-propeller domain in Kelch protein KLHL40 changes upon nemaline myopathy-associated mutation†
Abstract
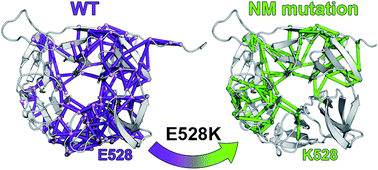
Evolutionarily widespread, functionally and structurally diverse and still largely unexplored, Kelch proteins, characterized by the presence of a conserved C-terminal β-propeller, are implicated in a number of diverse fundamental biological functions, including cytoskeletal arrangement, regulation of cell morphology and organization, and protein degradation. Mutations in the genes encoding for Kelch superfamily members are being discovered as the cause of several neuromuscular diseases and cancer. The E528K mutation in Kelch protein KLHL40, which regulates skeletal muscle myogenesis, has been identified as a frequent cause of severe autosomal-recessive nemaline myopathy (NM). We use all-atom molecular dynamics simulations to characterize the dynamic behaviour of the β-propeller of the wild-type protein and identify correlated motions underlying the in vivo functionality. We also modelled the NM-associated mutation and we found that it does not lead to dramatic disruption of the β-propeller architecture; yet, residue 528 is a hub in the correlated motions of the domain, and mutation-induced local structural alterations are propagated to the whole protein, affecting its dynamics and physicochemical properties, which are fundamental for in vivo interaction with partners. Our results indicate that rational design of drugs can be envisioned as a strategy for restoring the internal network of communication and resetting KLHL40 to its physiological state.

 Please wait while we load your content...
Please wait while we load your content...