Fabrication of a new modified gold electrode based on gold nanoparticles and nanomagnetic Fe3O4/SiO2–(CH2)3–SH core shell for electrochemical evaluation and determination of dinitramine herbicide in water†
Abstract
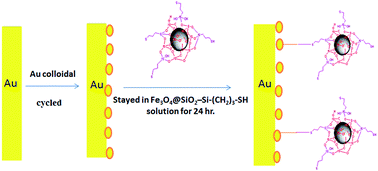
A novel and sensitive method is described for the voltammetric study and determination of dinitramine, a commonly used herbicide, based on its electrochemical oxidation on gold electrodes modified with gold nanoparticles and Fe3O4/SiO2–Si–(CH2)3–SH core shell (Au/Au-NPs–S–(CH2)3–Si–SiO2/Fe3O4). Cyclic voltammetry (CV) was used to investigate the electrochemical properties of dinitramine on this modified electrode at various solution pH values and various scan rates. CV studies indicated that the oxidation process has an irreversible and diffusion-like behavior in an oxidation mechanism with an equal number of electrons and protons. A possible reaction mechanism was proposed. Square-wave voltammetry (SWV) was applied as a very sensitive voltammetric detection method for the determination of dinitramine. Under optimal conditions, the proposed method exhibited acceptable analytical performances in terms of linearity (over the concentration range from 5.0 × 10−9 to 7.0 × 10−6 mol L−1, R2 = 0.997), detection limit (0.3 × 10−9 mol L−1) and repeatability (RSD = 1.13%, n = 10, for 1.0 × 10−7 mol L−1 dinitramine). To further validate its possible application, the method was used for the quantification of dinitramine in water samples.

 Please wait while we load your content...
Please wait while we load your content...