Exploring structural requirements for the inhibition of Plasmodium falciparum calcium-dependent protein kinase-4 (PfCDPK-4) using multiple in silico approaches
Abstract
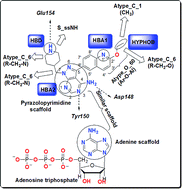
Plasmodial protein kinases represent one of the most important thrust areas for antimalarial drug discovery. Although kinases are difficult to target, Plasmodium calcium-dependent protein kinases (CDPKs) are attractive drug targets because they play an essential role in the life cycle of the malaria parasite while being absent in humans. Against this background, we made an attempt in the present study to decipher the structural requirements for the inhibition of P. falciparum calcium-dependent protein kinase-4 (PfCDPK-4). Here, we utilized a data set of 89 structurally diverse pyrazolopyrimidine- and imidazopyrazine derivatives for in silico studies involving quantitative structure activity relationship (QSAR), quantitative structure activity–activity relationship (QSAAR), 3D-pharmacophore, docking and molecular dynamics simulations. The QSAR model highlighted the contributions of atom-type log P for R–CH3, R4C and Al–O–Ar fragments in determining the inhibitory potency against PfCDPK-4. The model also suggested the role of lower electron density (lower number of π bonds and loan pair of electrons) and the involvement of singly bonded –NH fragments in predicting the inhibitory activity. The QSAAR model comprising both PfCDPK-1 and PfCDPK-4 activities reflected the importance of the inhibitory response (PfCDPK-1), the alkoxy-type oxygen fragment (Al–O–Ar), the secondary alkyl fragment (R–CH2–X), the singly bonded oxygen atom (–O–) and the total hydrophobic surface area in predicting the inhibitory activity against the PfCDPK-4 target. Pharmacophore models were developed to identify the requisite groups essential for the activity and to classify the PfCDPK-4 inhibitors into more active and less active classes. The best pharmacophore model (hypothesis-1) showed four pharmacophoric features, namely, two hydrogen bond acceptors (HBA), one hydrogen bond donor (HBD) and one hydrophobic (HYPHOB) feature with a correlation coefficient of 0.933. The model showed 85.71% and 75.41% classification accuracies for the training and test sets, respectively. The docking study revealed that the highest active PfCDPK-4 inhibitor interacts with the ATP pocket residues of Lys78, Glu197, Asp148 and Tyr150 through hydrogen bonding and ionic interactions. Stable H-bond (Glu154, Asp148, Tyr150) and hydrophobic (Val84, Ala97, Tyr150, Ile214) interactions observed during 10 ns simulation may be responsible for compound 72 possessing the highest inhibitory activity among all the pyrazolopyrimidine derivatives. The molecular dynamics studies also showed that the protein complex of the most active inhibitor is more stable than the complex of the least active inhibitor in the dynamic environment.

 Please wait while we load your content...
Please wait while we load your content...