Residue dependent hydrogen-bonding preferences in orthanilic acid-based short peptide β-turn motifs†
Abstract
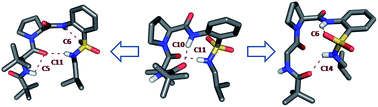
This communication describes the competition between native β-turn (C10) and 2-aminobenzenesulfonic acid (SAnt)(orthanilic acid)-based pseudo β-turn (C11) in their hybrid peptides. Solid-state crystal structure and solution-state NMR studies revealed that C10 and C11 can be simultaneously observed under appropriate conditions. The variable temperature NMR coefficient data suggest that the isolated C11/C14 hydrogen bond is weaker in comparison with the consecutive C10 and C11 turns.

 Please wait while we load your content...
Please wait while we load your content...