CuII immobilized on guanidinated epibromohydrin functionalized γ-Fe2O3@TiO2 (γ-Fe2O3@TiO2-EG-CuII): a novel magnetically recyclable heterogeneous nanocatalyst for the green one-pot synthesis of 1,4-disubstituted 1,2,3-triazoles through alkyne–azide cycloaddition in water†
Abstract
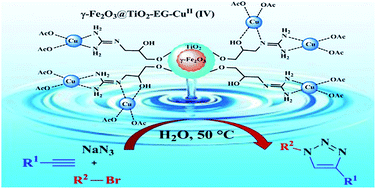
CuII immobilized on guanidinated epibromohydrin functionalized γ-Fe2O3@TiO2 (γ-Fe2O3@TiO2-EG-CuII) as a novel magnetically recyclable heterogeneous catalyst was synthesized and characterized by various techniques such as FT-IR, TGA/DTA, HRTEM, XRD, EDX, VSM, ICP and elemental analysis. The catalyst demonstrated a superior catalytic activity towards the green one-pot synthesis of 1,4-disubstituted 1,2,3-triazoles via the three-component reaction between terminal alkynes, organic bromides, and sodium azide, in water. This method avoids isolation and handling of organic azides, because of their in situ generation. Importantly, the synthesized catalyst was separated easily by using an external magnet and recycled for six runs without appreciable leaching of copper from the surface of the catalyst.

 Please wait while we load your content...
Please wait while we load your content...