Modulating the mixed micellization of CTAB and an ionic liquid 1-hexadecyl-3-methylimidazollium bromide via varying physical states of ionic liquid†
Abstract
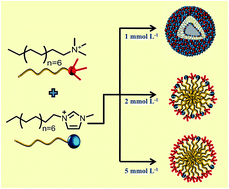
The mixed micellization behavior in aqueous systems of CTAB and an ionic liquid (IL), 1-hexadecyl-3-mthylimidazolium bromide, [C16mim][Br], has been investigated at different fixed concentrations of IL i.e. 1, 2 and 5 mmol L−1. The mixed micellization phenomenon has been investigated using conductivity and steady-state fluorescence measurements to get the critical micelle concentration (cmc) and aggregation number (Nagg) of formed mixed micelles. The insight into thermodynamic forces behind the mixed micellization has been obtained from isothermal titration calorimetry (ITC). The size and shape of the formed micelles has been investigated using dynamic light scattering (DLS) and transmission electron microscopy (TEM) measurements. The presence of IL in varying physical forms i.e. predominantly in monomeric form at 1 mmol L−1, in the micellar form at 2 mmol L−1 and rich in micelles at 5 mmol L−1 of IL is expected to modulate the formation of mixed micelles in aqueous medium. At different concentrations of IL, varying forms of self-assembled structures such as IL rich CTAB–IL mixed vesicles (1 mmol L−1) and IL rich CTAB–IL mixed micelles (2 and 5 mmol L−1) have been observed. 1H NMR measurements along with 1H spin–lattice relaxation time have shed light on the relative content of IL and CTAB in the formed mixed structures.

 Please wait while we load your content...
Please wait while we load your content...