Molecular dynamics simulation of the structural, elastic, and thermal properties of pyrochlores
Abstract
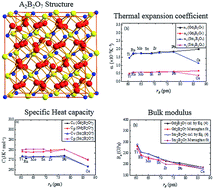
We present a comprehensive simulation study of the effect of composition on the structural, elastic and thermal properties of 25 different compounds from the pyrochlore family. We joined a repulsive potential to an existing interatomic potential to enable molecular dynamics simulations of conditions away from equilibrium. We systematically varied the chemistry of the pyrochlore by substituting different cations in the A and B sites of the A2B2O7 formula unit. The A cations varied from Lu3+ to La3+, and the B cations from Ti4+ to Ce4+. The lattice parameter increased steadily with increasing the radius of A or B cations, but the bulk modulus showed a decreasing trend with increasing cation radius. However, the specific heat capacity and thermal expansion coefficient remained almost unchanged with increasing the radii of A and B cations. It is of interest to note that Ce on the B site significantly reduces the specific heat capacity and thermal expansion coefficient, which could have implications for annealing of radiation damage in cerate pyrochlores. The present results are consistent with the experimental measurements, which suggests that these potentials are appropriate for studying the problem of interest, namely simulation of dynamical processes, radiation damage, and defect migration in pyrochlores.

 Please wait while we load your content...
Please wait while we load your content...