Effects of water and alcohols on the polymerization of furan during its acid-catalyzed conversion into benzofuran†
Abstract
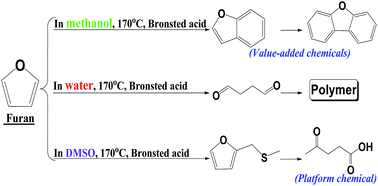
Furan, an important product from catalytic pyrolysis of biomass, has the potential to be further converted into value-added chemicals or biofuels. This study investigated the conversion of furan into benzofuran over a Brønsted acid catalyst (Amberlyst 70) at 140–190 °C in various solvents. With water as the solvent, furan could barely make its way to benzofuran as its polymerization dominated. With methanol as the solvent, the polymerization of furan was suppressed and benzofuran formation was enhanced substantially. This is because in methanol, the reactive intermediates (i.e., aldehydes) were stabilized and their involvement in polymerization reactions was suppressed. Other alcohols showed similar effects on suppressing polymerization. In dimethyl sulfoxide (DMSO), the polymerization of furan was also effectively suppressed. However, furan was not converted to benzofuran but to levulinic acid via a distinct reaction route.

 Please wait while we load your content...
Please wait while we load your content...