Ultrasensitive detection of sulfide ions through interactions between sulfide ions and Au(iii) quenching the fluorescence of chitosan microspheres functionalized with rhodamine B and modified with Au(iii)†
Abstract
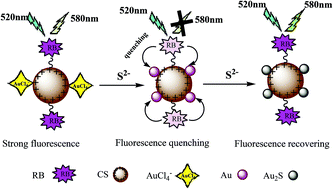
A rapid and facile fluorescence probe for detecting sulfide ions was developed. Sulfide is detected using the probe through interactions between sulfide ions and Au(III) quenching the fluorescence of chitosan microspheres functionalized with rhodamine B (RB-CSM) and modified with Au(III). The RB-CSM contain large numbers of positively charged amine groups and fluoresce strongly. Au(III) adsorb onto the RB-CSM surfaces through electrostatic interactions between AuCl4− and protonated amino groups in the RB-CSM, giving Au(III)/RB-CSM. The fluorescence of the Au(III)/RB-CSM is effectively quenched by sulfide ions through interactions between sulfide ions and Au(III), causing gold nanoparticles to form and allowing fluorescence resonance energy transfer between the gold nanoparticles and the RB-CSM to occur. Under optimum conditions, a good linear relationship (R2 = 0.982) was found between the fluorescence quenching efficiency (F0/F) and the sulfide ion concentration between 0.16 and 36 nmol L−1. The detection limit for sulfide ions was 0.1 nmol L−1. The probe can be completely regenerated using thiourea and was easily separated by centrifuging the mixture, meaning that the probe will cause much less environmental pollution than non-renewable fluorescence probes. The approach described here is a new and convenient way of developing reusable fluorescence probes.

 Please wait while we load your content...
Please wait while we load your content...