Ni/H-ZSM-5 as a stable and promising catalyst for COx free H2 production by CH4 decomposition†
Abstract
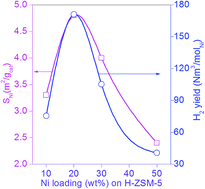
Catalytic decomposition of methane for COx free hydrogen production is carried out over Ni supported on H-ZSM-5 catalysts with different Si/Al ratios (i.e. 40, 150, 300 and 485) at 550 °C and atmospheric pressure. Methane decomposition activity of Ni/H-ZSM-5 is decreased with time on stream and finally deactivated completely. The fresh and reduced catalysts are characterized by BET-SA, XRD, FT-IR, UV-DRS, TPR, pulse chemisorption of H2 and N2O and some of the used catalysts are characterised by CHNS, SEM, TEM and Raman spectroscopy. Raman spectra of the used catalysts showed both ordered and disordered carbon at 1580 cm−1 and 1320 cm−1. The 20 wt% Ni/H-ZSM-5 (Si/Al = 150) exhibited a higher H2 production rate over the other Ni loadings. The superior performance of 20 wt% Ni/H-ZSM-5 (Si/Al = 150) is rationalized by the physico-chemical properties of the various Ni loaded H-ZSM-5 catalysts.

 Please wait while we load your content...
Please wait while we load your content...