Rapid and high-capacity adsorption of sulfonated anionic dyes onto basic bismuth(iii) nitrate via bidentate bridging and electrostatic attracting interactions†
Abstract
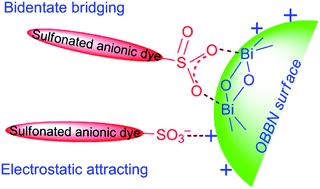
A new adsorbent of octahedron-structured basic bismuth(III) nitrate (OBBN), [Bi6O5(OH)3](NO3)5·3H2O, was synthesized by a mild hydrolysis route and used for the adsorption removal of sulfonated anionic dyes (SADs) from aqueous solutions. A typical SAD, methyl orange (MO), was taken to investigate the adsorption processes at different pH, adsorbent dosage, contact time, temperature and initial MO concentration. The equilibrium adsorption data were well fitted by the Langmuir isotherm model and showed high adsorption capacity (qmax = 1298 mg g−1). The adsorption kinetics was well described by the pseudo-second-order model and exhibited a short adsorption equilibrium time (<14 min for 20 mg L−1 MO). Adsorption thermodynamic parameters revealed that the adsorption is endothermic, random and spontaneous. The adsorption behavior was closely related to the combined interactions of bidentate bridging and electrostatic attraction between [Bi6O5(OH)3]5+ polycations on the OBBN surface and –SO3− groups of the SAD. The adsorbent was successfully applied to remove MO from model wastewater with a satisfactory result.

 Please wait while we load your content...
Please wait while we load your content...