Mycobacterium tuberculosis histidinol dehydrogenase: biochemical characterization and inhibition studies†
Abstract
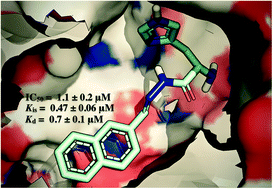
HisD-Encoded histidinol dehydrogenase (HisD) catalyzes the two last chemical reactions of the L-histidine biosynthetic pathway, namely the conversion of L-histidinol (L-Hol) to L-histidinaldehyde (L-Hal) and to L-histidine (L-His). The hisD gene product has been shown to be essential for Mycobacterium tuberculosis survival in vitro. Herein, we describe a series of biochemical studies on recombinant Mycobacterium tuberculosis HisD (MtHisD). The synthesis of hydrazones derived from L-histidine yielded inhibitors in the low micromolar range, one of which showed moderate anti-Mtb activity. The compounds described here are, to the best of our knowledge, the first inhibitors of MtHisD activity reported in the literature, and they could become promising candidates for future development.


 Please wait while we load your content...
Please wait while we load your content...