Iron and nickel co-doped cobalt hydroxide nanosheets with enhanced activity for oxygen evolution reaction†
Abstract
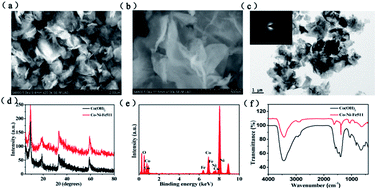
A poor oxygen evolution reaction (OER) activity restricts the efficiency of large scale H2 production from water splitting. Developing highly active, low cost and lasting electrocatalysts has become a top priority. Herein, we have successfully synthesized iron and nickel co-doped cobalt hydroxide (Co–Ni–Fe511) nanosheets for the OER. This catalyst exhibits outstanding performance with an overpotential of 288 mV at a current density of 10 mA cm−2, a high polarization current of about 67 mA cm−2 at 330 mV overpotential, a low Tafel slope of 43 mV dec−1, and small degradation after 8 hours of long-term OER operation. In addition, we simulate alkaline water electrolysis at a current density of 200 mA cm−2 at 70 °C in 6 M KOH electrolyte, and the energy consumption of Co–Ni–Fe511 is 45.7 kW h kgH2−1. For comparison, commercial RuO2 consumed 46.8 kW h kgH2−1. This suggests that Co–Ni–Fe511 is more appropriate than commercial RuO2 for water electrolysis in commercial water electrolysis. The high activity, low Tafel slope, good stability and low energy consumption will pave the way for the use of Co–Ni–Fe511 in practical applications.

 Please wait while we load your content...
Please wait while we load your content...