Revisiting oxime–nitrone tautomerism. Evidence of nitrone tautomer participation in oxime nucleophilic addition reactions†
Abstract
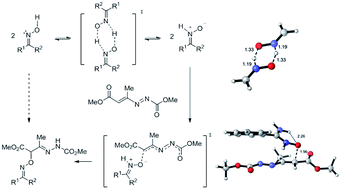
The oxime–nitrone tautomerism has been revisited using high-level DFT calculations. The isomerization has been found to be more favorable through a bimolecular process involving two molecules of oxime, a finding that argues against the commonly accepted thermal 1,2-H-shift mechanism. The reaction of arylamidoximes with 1,2-diaza-1,3-dienes to yield the corresponding O-substituted oximes (stable intermediates for the synthesis of 1,2,4-oxadiazine derivatives) was also investigated as a rare case in which O-alkylation is observed in the reaction between oximes and electron-poor alkenes in the absence of a base. Under such conditions the reaction usually proceeds through the nucleophilic attack of the oxime nitrogen to yield the corresponding nitrone. The computational investigation revealed that in the case of arylamidoximes, the pathway involving the less stable but more reactive nitrone tautomer is the predominant mechanism, evidencing for the first time the involvement of a nitrone tautomer in nucleophilic additions of oximes. Validation of the model has been carried out by studying alternative ene-like processes; the dramatically different reactivity predicted for arylamidoximes and unsubstituted oxime are rationalized in terms of steric hindrance.

 Please wait while we load your content...
Please wait while we load your content...