Aromatic-like behavior of germanium nanocrystals
Abstract
Based on density functional theory calculations, aromatic germanium nanocrystals Ge19H12, which are composed of three parallel, planar hexagons with one additional Ge atom close to the cluster center, have been predicted. By exploring electron deficiency and electron delocalization in the proposed nanocrystals, the definition of the electron-deficiency aromaticity concept is extended to germanium nanocrystals. The natural bond orbital analysis and nucleus independent chemical shifts have been used to investigate the electron delocalization in the nanocrystals. We show new patterns for the optical spectrum of Ge19H12 which may be attributed to the special electronic structure of the studied nanoparticles. This study reports relevant details for the synthesis and structuring of overcoordinated germanium nanocrystals and deserves further work for the development of germanium-based nanoparticles for energy and microengineering purposes.
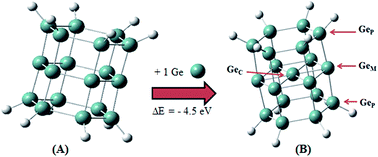

 Please wait while we load your content...
Please wait while we load your content...