Effect of cerous phosphates with different crystal structures on their acidity and catalytic activity for the dehydration of glucose into 5-(hydroxymethyl)furfural†
Abstract
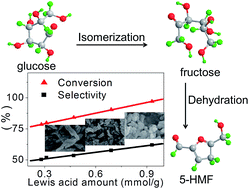
A series of cerous phosphate (CP) catalysts with different crystal structures were synthesized by a hydrothermal method at different temperatures (120, 140, 160, 180 and 200 °C) and their performances for the dehydration of glucose into 5-(hydroxymethyl)furfural (HMF) were also thoroughly investigated. These catalysts were characterized by XRD, N2 adsorption–desorption, SEM, in situ DRIFT, NH3-TPD and XPS. The results indicate that changing the temperature of synthesis will lead to a transformation of the crystal phase and morphology from 120 °C (nanoparticles, hexagonal structure) to 200 °C (nanorods, monoclinic structure); also, the different crystal phases possess different surface Ce4+ amounts and acidities. A good linear correlation is found between the Lewis acid content and the surface Ce4+ amount among these CP catalysts, and very good linearity is also displayed between the Lewis acid amount and the conversion of glucose or the selectivity of HMF, which indicates that Lewis acidity plays an important role in the dehydration of glucose to HMF. CP120, which has a hexagonal crystal structure, exhibits the best catalytic activity (97% conversion of glucose and 61% yield of HMF) because it has the highest amounts of Lewis acid and total acid.

 Please wait while we load your content...
Please wait while we load your content...