Degradation of tetrabromobisphenol A in heat activated persulfate oxidation process†
Abstract
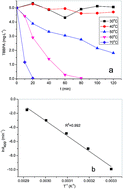
Sulfate radicals (SO4˙−) generated by heat activated persulfate were employed to degrade brominated flame retardant tetrabromobisphenol A (TBBPA). Experimental results showed that the reaction rate was first order to the concentrations of both persulfate and TBBPA. The degradation of TBBPA was accelerated with increasing temperature. Radical scavenging tests using ethanol and t-butanol as probes revealed that SO4˙− was the dominant oxidizing species. The degradation efficiency was adversely affected by the presence of humic acid and HCO3−. No significant inhibition of TBBPA degradation was observed in the presence of Cl−. Seven brominated intermediates and products were identified by liquid chromatography-mass spectrometry. Based on this, debromination and electron transfer followed by β-scission were proposed to be the primary pathways of TBBPA degradation. The results of this study suggest that the heat activated persulfate process could be an effective approach to remove TBBPA in aqueous solution.

 Please wait while we load your content...
Please wait while we load your content...