A facile and efficient method for the synthesis of alkynone by carbonylative Sonogashira coupling using CHCl3 as the CO source†
Abstract
A facile and efficient method for the synthesis of alkynones by a Pd-catalyzed carbonylative Sonogashira coupling reaction starting from aryl iodide, terminal alkyne and chloroform (CHCl3) as the CO source is described. This procedure proves that CHCl3 is a cheap and efficient CO source in the presence of CsOH·H2O as the base. Furthermore, it is applied successfully for the modification of natural products, such as vindoline and tabersonin, to obtain the corresponding products in good yields.
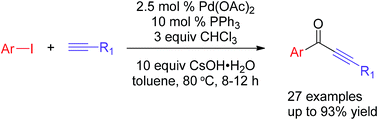
- This article is part of the themed collection: Editors’ collection: Catalytic Organic Transformations

 Please wait while we load your content...
Please wait while we load your content...