Highly enhanced leukemia therapy and oral bioavailability from a novel amphiphilic prodrug of cytarabine†
Abstract
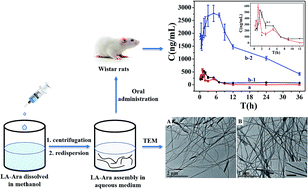
Cytarabine (1-β-D-arabinofuranosylcytosine, Ara-C), an attractive medicinal treatment for myeloblastic leukemia, is subject to low oral bioavailability due to its weak membrane permeability and lipophilicity as well as its poor metabolic stability. Based on these considerations, we proposed a chemical linkage of cytarabine with lauric acid (LA), a long-chain fatty acid with 12 carbons, leading to a new prodrug, LA–Ara. The NH2 group of Ara-C is protected by conjugation and thus cannot not be deactivated by deamination; moreover, notably high liposolubility and penetrability were obtained. When dispersed in water, this new amphiphilic molecule can adopt a nanofiber configuration. It was found that LA–Ara molecules are stable in artificial biological media, indicating that the prodrug could be administrated orally. MTT assays on HL60 and K562 cells were performed, and the significantly higher cytotoxicity compared to the pure drug suggested that the prodrug has conspicuously superior antiproliferative activity. Following oral administration, the elimination half-life and bioavailability of the LA–Ara group dramatically increased to 6.6- and 32.8-fold of those of free Ara-C, respectively. It appears that this new prodrug could be an effective oral alternative treatment to Ara-C and supply a promising therapy index for leucocythemia.

 Please wait while we load your content...
Please wait while we load your content...