Highly stable organic–inorganic junction composed of hydrogenated titania nanotubes infiltrated by a conducting polymer†
Abstract
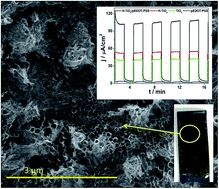
A poly(3,4-ethylenedioxythiophene) conducting polymer doped with poly(2-styrene sulfonate) (pEDOT:PSS) was efficiently electrodeposited on a layer composed of ordered titania nanotubes. TiO2 nanotubes were formed during an anodization process and, after calcinations, a layer was subjected to hydrogen plasma. Hydrogenation leads to Ti(III) formation, a decrease in resistance, and a huge increase of donor density when compared with pure titania. According to a detailed structure analysis, the coverage by the polymer matrix is uniform on the entire titania surface as well as along the tubes. The composite material exhibits highly enhanced anodic photocurrent (106 μA cm−2) when compared with hydrogenated titania H–TiO2 (54 μA cm−2) or pure polymer film (2 μA cm−2). Moreover, H–TiO2/pEDOT:PSS is characterized with high photostability displayed during prolonged illumination. The proposed hydrogenation approach could be regarded as a facile titania modification for further electrochemical modifications.

 Please wait while we load your content...
Please wait while we load your content...