Simple electrochemical synthesis of an Au–Ag–Cu trimetallic nanodendrite and its use as a SERS substrate†
Abstract
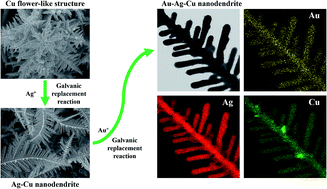
An Au–Ag–Cu trimetallic nanodendrite was constructed by simple electrochemical methods and was evaluated as a surface enhanced Raman scattering (SERS) substrate. The motivation was to harmonize the individual merits of each SERS-active metal, the higher SERS efficiency of Ag, good surface stability of Au, and structural stability of Cu, in a single structure. For the fabrication, a Cu flower-like nanostructure was initially constructed by reduction of Cu2+ (electrodeposition). Next, a galvanic replacement reaction (GRR) was performed on the Cu framework in a solution of Ag+ to build Ag nanodendrites with coincidental release of Cu atoms into the solution as Cu2+. As the Ag nanodendrite grew, released Cu2+ redeposited, so a Ag–Cu bimetallic nanodendrite started to form. Next, a second GRR was performed with the newly prepared Ag–Cu nanodendrite in Au3+ solution to partially replace Ag and Cu by Au to construct the Au–Ag–Cu trimetallic nanodendrite. Based on examination of Raman peaks of rhodamine 6G (R6G, a reporter molecule), the Ag–Cu nanodendrite resulted in approximately 2.6 fold higher peak intensity compared to a Ag nanodendrite. Subsequent Au incorporation into the Ag–Cu nanodendrite greatly improved the stability of SERS measurements as well as increased the peak intensity further by 7.6 fold, thereby enabling the observation of Raman peaks of a 10−10 M R6G sample.

 Please wait while we load your content...
Please wait while we load your content...