Adsorption of berberine hydrochloride onto mesoporous carbons with tunable pore size†
Abstract
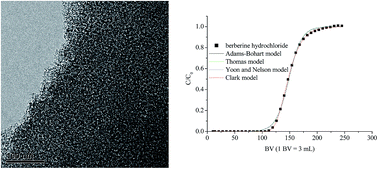
Eleven mesoporous carbon adsorbents were synthesized and characterized, and the adsorption properties of berberine hydrochloride on these adsorbents were investigated. The mesoporous carbon adsorbents have high BET specific surface areas (1051.2 to 1554.9 m2 g−1), large pore volumes (1.42 to 2.31 m3 g−1), and their average pore radii range from 1.53 nm to 3.28 nm. The adsorption capacities of berberine hydrochloride on these adsorbents strongly depend on the BET specific surface areas and pore volumes of the mesoporous carbons. The mesoporous carbon sample with the highest BET specific surface area was selected as the most promising adsorbent for adsorption of berberine hydrochloride because of its high adsorption capacities (415 mg g−1 at 298 K, 0.10 mg mL−1). The adsorption thermodynamic parameters of berberine hydrochloride on the selected adsorbent were calculated. The adsorption equilibrium of berberine hydrochloride on the selected adsorbent could be established within 240 minutes at 298 K. The dynamic adsorption capacity of berberine hydrochloride on the selected adsorbent is close to the theoretical maximum adsorption capacity; and 72.4% of the adsorbed berberine hydrochloride could be desorbed by a 70% alcohol aqueous solution. The excellent adsorption/desorption properties make the mesoporous carbons promising adsorbents for adsorption of berberine hydrochloride from aqueous solutions.

 Please wait while we load your content...
Please wait while we load your content...