Gold/gallium-catalyzed annulation of 1,3-dicarbonyl compounds and cyclopropylacetylenes for synthesis of substituted cyclopentenes†
Abstract
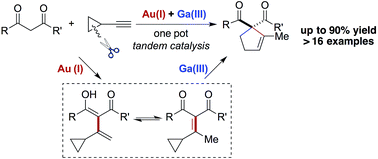
An efficient synthesis of substituted cyclopentenes through gold/gallium-catalyzed annulation of 1,3-dicarbonyl compounds and cyclopropylacetylenes is achieved. This tandem reaction consists of a gold-catalyzed Nakamura reaction, a catalytic dienol–enone tautomerization, and a gallium-catalyzed vinylcyclopropane (VCP) rearrangement.

 Please wait while we load your content...
Please wait while we load your content...