Abstract
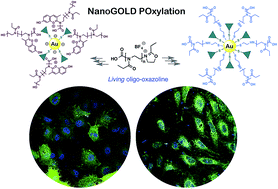
Gold nanoparticles (GNPs) are one of most investigated nanomaterials for lung cancer diagnosis and therapy (theragnosis). For imaging purposes, GNPs are often tagged with fluorescent probes, but unfortunately the associated plasmon resonance effect leads to fluorescence self-quenching, thus precluding accurate localization. In this study, biocompatible GNPs targeted with a laminin fragment were successfully engineered using fluorescent oligo-oxazolines produced in supercritical carbon dioxide. The architecture and properties of the POxylated constructs were fully characterized and confocal laser scanning microscopy measurements demonstrated a higher cellular uptake into A549 lung cancer cells through an active targeting mechanism.


 Please wait while we load your content...
Please wait while we load your content...