In situ generation and trapping of thioimidates: an intermolecular tandem reaction to 4-acylimino-4H-3,1-benzothiazines†
Abstract
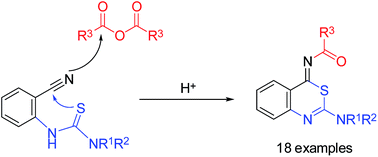
The proton-catalyzed transformation of 2-thioureidobenzonitriles to 4-acylimino-4H-3,1-benzothiazines was accomplished by treatment with carboxylic anhydrides, acid chlorides or alkyl chloroformates. The reaction involves a cyclization to 4-imino-3,1-benzothiazinium salts, whose thioimidate structure is trapped by a subsequent reaction with the acyl donor. 2-Ureidobenzonitriles do not undergo such an intermolecular tandem reaction. The different reaction behavior of both types of substrates was verified by NMR monitoring and employing 18O-enriched water.

 Please wait while we load your content...
Please wait while we load your content...