Construction of a 2D/2D g-C3N4/rGO hybrid heterojunction catalyst with outstanding charge separation ability and nitrogen photofixation performance via a surface protonation process
Abstract
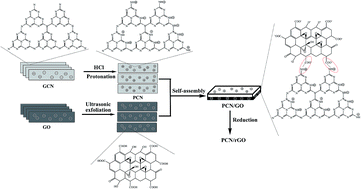
In this work, we report a 2D/2D hybrid heterojunction photocatalyst (PCN/rGO) with effective interfacial contact by incorporating reduced graphene oxide (rGO) and protonated g-C3N4 (PCN) synthesized via a novel electrostatic self-assembly strategy, followed by an ethylene glycol reduction process. The GCN/rGO obtained by incorporating reduced graphene oxide (rGO) and g-C3N4 without protonation (GCN) has also been prepared for comparison. PCN/rGO(0.2), with the mass ratio rGO/PCN of 0.2, exhibits the highest nitrogen photofixation performance under visible light. The NH4+ generation rate for PCN/rGO(0.2) is 42.4, 8.3 and 3.7 times higher than that of GCN, PCN and GCN/rGO(0.2). This is ascribed to the addition of rGO with PCN in a controlled ratio as well as sufficient interfacial contact by the electrostatic self-assembly process between rGO and PCN across the PCN/rGO heterojunction, for efficient charge transfer to suppress the recombination of electron–hole pairs, as evidenced by the zeta potential, XPS and PL studies. In addition, compared with GCN/rGO(0.2), the stronger interaction caused by the electrostatic attractive forces exists between PCN and rGO, leading to a better catalytic stability of PCN/rGO(0.2). This work opens up an effective way to prepare the heterojunction catalyst by incorporating two components with different surface charges.

 Please wait while we load your content...
Please wait while we load your content...