Ca(OH)2 nano-pods: investigation on the effect of solvent ratio on morphology and CO2 adsorption capacity
Abstract
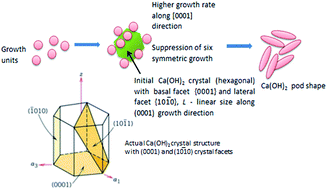
Ca(OH)2 nano-pods were synthesized through a precipitation method. Solvents such as ethanol/deionized water (DIW) and dimethylformamide (DMF)/deionized water (DIW) were used at different volume ratios to synthesize the samples. Various characterization techniques such as X-ray diffraction (XRD), filed emission scanning electron microscopy (FESEM), high resolution transmission electron microscopy (HRTEM) and BET surface area analysis were employed to investigate the role of solvent on the crystallinity, morphology and surface area of Ca(OH)2. The solvent mixtures with a high volume of organic solvent (ethanol or DMF) acted as good capping agents to suppress the growth of Ca(OH)2 in the (1010) direction and induce anisotropic growth along the (0001) direction. A uniform pod like morphology was observed for the Ca(OH)2 sorbent synthesized using ethanol/DIW with a volume ratio of 78 ml/02 ml. Besides, the sorbents synthesized using ethanol/DIW showed good CO2 adsorption capacity and high surface area when compared to that of DMF/DIW.

 Please wait while we load your content...
Please wait while we load your content...