Synthesis and photovoltaic properties of a 2D-conjugated copolymer based on benzodithiophene with alkylthio-selenophene side chain†
Abstract
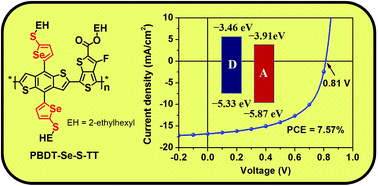
A new 2D-conjugated copolymer (PBDTSe-S-TT) based on alkylthio-selenophene substituted benzodithiophene (BDTSe-S) and 2-ethylhexyl 4,6-dibromo-3-fluorothieno[3,4-b]thiophene-2-carboxylate (TT) was designed and synthesized for application as a donor material in polymer solar cells (PSCs). PBDTSe-S-TT shows a broad absorption in the wavelength range from 300 to 800 nm, and a lower highest occupied molecular orbital (HOMO) energy level of −5.33 eV. The PSCs based on PBDTSe-S-TT as donor and [6, 6]-phenyl-C71-butyric acid methyl ester (PC71BM) as acceptor with 3% DIO as additive exhibited a power conversion efficiency (PCE) of 7.57%, under the illumination of AM 1.5 G, 100 mW cm−2. These results indicate that attaching an alkylthio-selenophene side chain in 2D-conjugated polymers could be an alternative method to enhance the Voc and PCE of the PSCs.

 Please wait while we load your content...
Please wait while we load your content...