Novel ratiometric turn-on fluorescent probe for selective sensing of cyanide ions, effect of substitution and bio-imaging studies†
Abstract
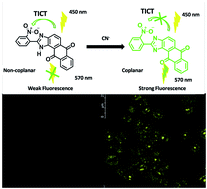
Novel fluorogenic receptors S1–S4 (imidazo anthraquinone derivatives) were synthesized from o-substituted benzaldehyde and 1,2-diaminoanthraquinone and characterised with various spectroanalytical techniques. In the case of S1, the selectivity towards cyanide ions with turn-on fluorescence output among other anions, due to the inhibition of the twisted intramolecular charge transfer (TICT) mechanism. The effect of substitution was successfully compared with sensing studies of S2–S4. S1 showed a good binding constant with a 1 : 1 stoichiometric ratio and micromolar detection limit. The sensing behaviour was further supported by 1H NMR titration, TD-DFT calculations and lifetime measurement studies. S1 was applied for the bio-imaging of the cell line RAW264.7 and successfully sensed cyanide ions under physiological conditions.


 Please wait while we load your content...
Please wait while we load your content...