Photocatalysis from a series of polyoxoazocobaltate high-nuclearity nanoclusters†
Abstract
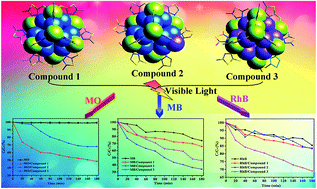
Three {Co20} nanoclusters possessing photocatalytic activity, namely, [Co20(OH)24(MMT)12(SO4)](NO3)2·6H2O (1), [Co20(MT)12(μ3-OH)23(μ3-O)(SO4)(CH3O)]·2EtOH (2) and [Co20(AMT)11(MT)(μ3-OH)22(μ3-O)2(SO4)(H2O)]·EtOH·4H2O (3) (MMT = 2-mercapto-5-methyl-1,3,4-thiadiazole, MT = 2-mercapto-1,3,4-thiadiazole, AMT = amino-5-mercapto-1,3,4-thiaoldiaze and EtOH = ethanol) have been obtained under hydrothermal conditions. Compound 1 presents a homometallic CoII cluster containing the α-Keggin polyoxometalate structure. Compound 2 features a cobalt cluster including the α-Keggin-type polyoxoazocobaltate {Co12} core, capped by seven octahedral CoII ions and one square-pyramidal CoII ion. Different from compounds 1 and 2, compound 3 possesses the semi-open square-pyramidal-based {Co10} core, and ten octahedral CoII ions are distributed around the core cluster. Through the comparison from structures of compound 1 and compounds 2–3, the different substituent groups of the organic ligands play an important role in the formation of the final homometallic CoII clusters. The electrochemical behaviors of the title compounds at room temperature have been investigated. Moreover, the high-nuclearity metal clusters are firstly acted as photocatalysts to be researched, and then the photocatalytic properties show that compounds 1–3 as the potential photoactive materials have the selectivity for degradation of some organic dyes.

 Please wait while we load your content...
Please wait while we load your content...