Acid-promoted rapid solvent-free access to substituted 1,4-dihydropyridines from β-ketothioamides†
Abstract
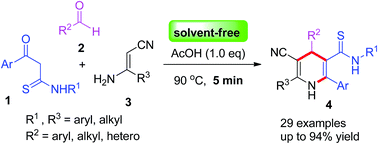
β-Ketothioamides (KTAs) have been used as building blocks with aldehydes and β-enaminonitriles for synthesis of 1,4-dihydropyridines in the presence of AcOH under solvent-free conditions within 5 min. This new strategy exhibits remarkable features such as high chemoselectivity, mild reaction conditions, easily available substrates, and good yields.

 Please wait while we load your content...
Please wait while we load your content...