Colorimetric detection of lead(ii) ions based on accelerating surface etching of gold nanorods to nanospheres: the effect of sodium thiosulfate
Abstract
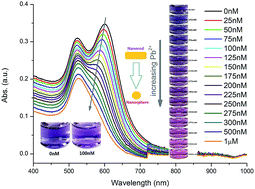
A non-aggregation colorimetric sensing method based on the accelerated etching of gold nanorods (AuNRs) has been developed for detecting lead(II) ions (Pb2+). In this method, the addition of Pb2+ leads to the formation of a monolayer of AuPb2 and AuPb3 alloys on the gold surface, which results in a considerable decrease in the surface electrode potential. Therefore, the sodium thiosulfate induced dissolution rate of AuNRs has been accelerated. Because of this accelerated etching, the shape of the AuNRs changes rapidly to gold nanospheres. This morphology transformation leads to a blue shift and fade down of the longitudinal localized surface plasmon resonance (LSPR) absorption band of AuNRs until it merges into the transversal absorption. The qualitative spectral change from double bands to single band LSPR results in a distinct irreversible color change in the gold colloid from blue to red. This colorimetric sensing method can be used to detect Pb2+ with excellent selectivity and high sensitivity. Under the optimized conditions, the lowest limit of detection for Pb2+ observed by the naked eye is 0.1 μM and 20 nM measured by UV-Vis spectroscopy. In the concentration range from 25 to 300 nM of Pb2+, this sensing method exhibits a good linear relationship. This colorimetric sensing method for Pb2+ has promising applications in water and human serum analysis.

 Please wait while we load your content...
Please wait while we load your content...