Controlled synthesis of CaTiO3:Ln3+ nanocrystals for luminescence and photocatalytic hydrogen production†
Abstract
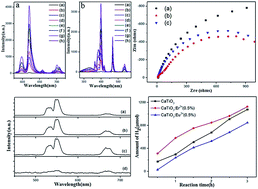
Bifunctional CaTiO3:Ln3+ (Ln = Eu and Er) nanocrystals were prepared via a facile method followed by calcination in air. The as-prepared CaTiO3:Ln3+ nanocrystals exhibited bifunctional performance for both photoluminescence and photocatalytic hydrogen production. As a phosphor powder, the luminescence properties of CaTiO3:Ln3+ nanocrystals could be controlled by doping with different Ln3+ ions. They showed very stable luminescence properties and a much higher quenching concentration due to the scheelite related structure of CaTiO3, which was up to 17% (Eu doping). As a photocatalyst, the CaTiO3:Ln3+ nanocrystals exhibited a higher activity for hydrogen production under ultraviolet light irradiation. The CaTiO3:Er3+ nanocrystals displayed the highest photocatalytic activity of up to 461.25 μmol h−1, which was higher than that of CaTiO3:Eu3+ nanocrystals and pure CaTiO3. The results indicated that incorporation of Ln3+ ions benefits electron transfer as well as the reduction of the band gap of the CaTiO3 photocatalyst.

 Please wait while we load your content...
Please wait while we load your content...