Inherently chiral heterocyclic resorcinarenes using a Diels–Alder reaction†
Abstract
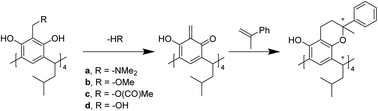
This paper presents a novel approach to highly diastereoselective synthesis of resorcinarenes having enlarged cavities. Inherently chiral heterocyclic resorcinarenes have been obtained with unusually high diastereoselecivity by a “one pot” sequence involving thermal generation of o-quinomethide resorcinarene derivatives and the subsequent Diels–Alder reaction with dienophiles (exemplified by α-methylstyrene). The diastereoselectivity of this reaction is explained based on the thermodynamic stability of the products. The enantiomers of rac-4 have been separated by HPLC and their absolute configuration was assigned by comparison of their experimental and theoretical CD spectra.

 Please wait while we load your content...
Please wait while we load your content...