Superparamagnetic nalidixic acid grafted magnetite (Fe3O4/NA) for rapid and efficient mercury removal from water†
Abstract
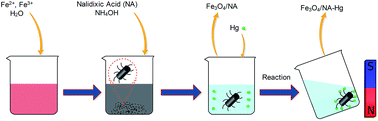
A new nanomaterial, nalidixic acid grafted magnetite (Fe3O4/NA), was synthesized via a chemical reaction with nano sized magnetite particles. The Fe3O4/NA was superparamagnetic at room temperature and could be separated by an external magnetic field. The presence of mercury in groundwater in wide scale industrial areas of the world has been a huge problem and the prepared Fe3O4/NA nanoparticles showed a high adsorption capacity towards Hg(II) as compared to bare magnetite particles. The high adsorption capacity of NA grafted Fe3O4 (9.52 mg g−1) was due to the increased adsorption sites in the magnetite-nalidixic acid (Fe3O4/NA). The sorption equilibrium data obeyed the Langmuir model while kinetic studies demonstrated that the sorption process of Hg(II) followed well the pseudo second order model. Since the Fe3O4/NA showed (over 99.8%) removal of the initial 1000 ppb Hg(II) within 60 min, it should be practically usable for Hg(II) contaminated water. The desorption of Hg(II) loaded on Fe3O4/NA could be successfully achieved with 0.001 M HCl containing 0.3 M thiourea, and the sorbent exhibited excellent reusability.


 Please wait while we load your content...
Please wait while we load your content...