Nitrosation and analysis of amino acid derivatives by isocratic HPLC
Abstract
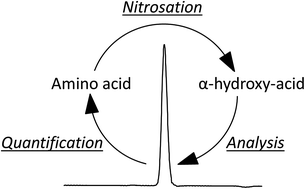
The objective of this study was to characterize the nitrosation of the classical amino acids by N2O3. Nitrosation of amino acids results in the formation of mainly α-hydroxy-acids that are suitable for isocratic HPLC analysis and subsequent quantification of amino acids in biological samples. The method is particularly suitable for detection of amino acids in e.g. fermentation media as the α-hydroxy-acids can be quantified in parallel to a variety of other organic substrates and products. The amino acids were transformed into their corresponding α-hydroxy-acids in acidic KNO2 solutions. The reactions were terminated by NaOH addition and the α-hydroxy-acids separated by isocratic HPLC and quantified by refractive index or UV absorption detection. Nitrosation of 18 of the classical amino acids; glycine, L-alanine, L-valine, L-leucine, L-isoleucine, L-methionine, L-serine, L-threonine, L-asparagine, L-glutamine, L-aspartic acid, L-glutamic acid, L-proline, L-cysteine, L-phenylalanine, L-lysine, L-tyrosine, and L-tryptophane formed detectable nitrosation products. L-Lysine, however, needed incubation in 96 mM formic acid to produce a detectable product, while L-phenylalanine had to be incubated in 120 mM HNO3 and 100 mM HCl. Optimal reaction conditions for most amino acids included 40 min of incubation of up to 5 g L−1 amino acid in 160 mM KNO2 in 100 mM HCl at 45 °C to maximize product yields.

 Please wait while we load your content...
Please wait while we load your content...