MnO2/TiO2 catalyzed synthesis of coenzyme pyridoxamine-5′-phosphate analogues: 3-deoxypyridoxamine-5′-phosphate†
Abstract
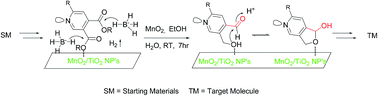
The highly efficient-selective synthetic route of the pyridoxal-5′-phosphate (vitamin B6, PLP) analogues: C3 substituted deoxy derivatives of pyridoxal (PL), pyridoxal-N-oxide (PLNO), pyridoxamine (PM), pyridoxamine-5′-phosphate (PMP) and pyridoxal-5′-phosphate (PLP), were developed via reduction and followed by selective oxidation in one pot using solid supported nanoparticles. The salient features of this strategy are: economic two-fold conversion, more Lewis acid sites, vacancies on the nanoparticle surfaces, and good yields of 3-deoxypyridoxal and 3-deoxypyridoxamine-5′-phosphate.

 Please wait while we load your content...
Please wait while we load your content...