Re-engineering of PIP3-antagonist triazole PITENIN's chemical scaffold: development of novel antifungal leads†
Abstract
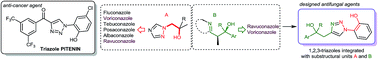
A novel 4-(1-phenyl-1-hydroxyethyl)-1-(o-hydroxyphenyl)-1H-1,2,3-triazole was designed by integrating the structural features of triazole PITENIN anticancer agents and the azole class of antifungal drugs. A two-step protocol comprising the Barbier propargylation and Cu-catalyzed azide–alkyne cycloaddition was established to synthesise a diverse set of compounds of this class. Their screening against a wide range of human fungal pathogens led to identification of several potential antifungal hits and some of them displayed better antifungal activity than fluconazole against Candida glabrata, Cryptococcus neoformans, Aspergillus fumigatus and Aspergillus niger. Mode of action studies revealed that their antifungal activity was resulting either from the inhibition of lanosterol 14 α-demethylase enzyme (leading to ergosterol depletion) or by the generation of reactive oxygen species (ROS).

 Please wait while we load your content...
Please wait while we load your content...