Pseudo-first order reaction kinetics and thermodynamic properties study of neem oil esterification using MgO grafted natural hydroxyapatite
Abstract
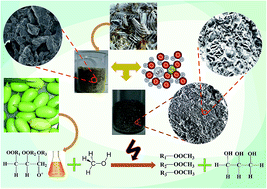
In this work, waste fish bone was used as a source of natural hydroxyapatite which was later used for the preparation of a metal grafted catalyst. Calcium phosphate is the main component of fish bone which has a relatively high catalytic activity, good thermal and chemical stability, and can be used for various applications. Magnesium oxide (MgO) was used for grafting due to its high specific surface area. It can be used as the active phase during catalysis which influences the selectivity and activity of catalytic reactions. In the present investigation, the waste fish bone-derived catalysts were characterized in detail and used for the esterification of neem oil (Azadirachta indica). The highest methyl ester yield of 96.7% was obtained at a 1 : 15 oil to methanol ratio, 70 °C reaction temperature and 6 wt% catalyst loading. The catalytic effect on yield and reusability of the catalyst was also studied. Subsequently, a kinetic model was developed and the thermodynamic properties of the process are illustrated. Overall the whole work gives a new direction towards the development of heterogeneous catalysts from bio wastages.

 Please wait while we load your content...
Please wait while we load your content...