Predicting 1,3,5,7-tetrakis(4-aminophenyl)adamantine based covalent-organic frameworks as hydrogen storage materials
Abstract
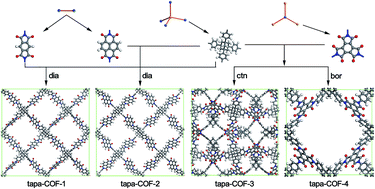
Four types of 1,3,5,7-tetrakis(4-aminophenyl)adamantine based covalent-organic frameworks (tapa-COFs) have been designed with diamond, ctn and bor net topologies using the methods of molecular mechanics and density function theory. Their low density (0.096–0.258 g cm−3), high porosity (90–96%) and large H2 accessible surface area (5511–6810 m2 g−1) forecast excellent hydrogen uptake capacities. The grand canonical Monte Carlo (GCMC) simulation revealed that at 77 K tapa-COF-1 possesses the highest gravimetric hydrogen storage capacity at 49.10 wt%, while tapa-COF-3 has the highest volumetric hydrogen storage capacity at 58.66 g L−1. Impressively, at 298 K, tapa-COF-1 and tapa-COF-2 possess rather high gravimetric hydrogen uptake capacities, which exceed both the U.S. Department of Energy’s goal (5.5 wt%) for onboard light-duty vehicles for 2020 and the criterion of 6 wt% for commercial use of hydrogen at room temperature. In addition, the possible schemes are also proposed to synthesize the tapa-COFs.

 Please wait while we load your content...
Please wait while we load your content...