Synthesis and properties of the derivatives of triphenylamine and 1,8-naphthalimide with the olefinic linkages between chromophores†
Abstract
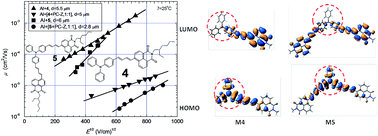
Two donor–acceptor type molecules consisting of triphenylamine and 1,8-naphthalimide moieties with the olefinic linkages between chromophores were synthesized by Heck reaction. The compounds obtained are capable of forming molecular glasses with glass transition temperatures of 56 and 75 °C recorded for mono- and di-substituted derivatives of triphenylamine, respectively. They exhibit high thermal stabilities with 5% weight loss temperatures of 350 and 363 °C. Fluorescence quantum yields of the dilute solutions of the synthesized compounds range from 0.065 to 0.72 while those of the solid films are 0.028 and 0.034. The Stokes shifts increased with the increase of the solvent polarity. Cyclic voltammetry measurements revealed close values of the solid state ionization potentials (5.22 and 5.27 eV) and of electron affinities (−3.20 and −3.18 eV). For the layer of the monosubstituted derivative of triphenylamine core hole mobility was found to be 2.1 × 10−3 cm2 V−1 s−1. Good intrinsic hole transport parameters were theoretically estimated in the frame of Marcus theory, and the impact of polaron-type hole transport in these materials is discussed.

 Please wait while we load your content...
Please wait while we load your content...