3-(5-(Benzylideneamino)thiazol-3-yl)-2H-chromen-2-ones: a new class of alkaline phosphatase and ecto-5′-nucleotidase inhibitors†
Abstract
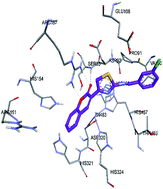
A new series of 3-(2-(benzylideneamino)thiazol-4-yl)-2H-chromen-2-ones has been synthesized. The structures of the compounds were established by means of 1H and 13C NMR spectroscopy. All compounds were evaluated for their potential to inhibit human recombinant ecto-nucleotidases, including human tissue-nonspecific alkaline phosphatase (h-TNAP), tissue specific human intestinal alkaline phosphatase (h-IAP), human and rat ecto-5′-nucleotidase (h-e5′NT & r-e5′NT). All the compounds were found to be potent and selective inhibitors of h-e5′NT, the most active h-e5′NT inhibitors were compounds 7a (IC50 = 0.25 ± 0.07 μM) and 7g (IC50 = 0.28 ± 0.05 μM). Most of the compounds were found to selectively inhibit h-TNAP over h-IAP, with inhibitory activities in the lower micromolar range. The most active h-TNAP inhibition was exhibited by compounds 7h and 7c (IC50 = 0.21 ± 0.04 μM and 0.22 ± 0.03, respectively), which is ≈91 times greater than the inhibitory activity of the standard inhibitor levamisole. Compound 7i was found to be the most potent h-IAP inhibitor (IC50 = 0.05 ± 0.001 μM), exhibiting ≈11 times greater selectivity for h-IAP over h-TNAP. Homology modeling, molecular docking and dynamics studies were carried out to determine the most plausible binding site interactions of potent inhibitors with ecto-nucleotidases.

 Please wait while we load your content...
Please wait while we load your content...