Hetero aromatic donors as effective terminal groups for DPP based organic solar cells†
Abstract
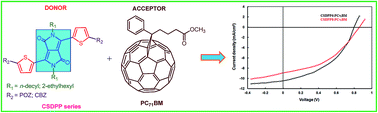
Four new solution-processable donor–acceptor–donor (D–A–D) structured low bandgap small molecules (CSDPP5, CSDPP6, CSDPP7 and CSDPP8) with diketopyrrolopyrrole as central acceptor unit and phenoxazine (POZ) or carbazole (CBZ) as terminal units were designed, synthesized and characterized. The new small molecules have been employed as donors along with the PC71BM as electron acceptor in solution processed BHJ organic solar cells, showed broad absorption bands with suitable electrochemical energy levels. When the BHJ active layer was cast from THF solvent, the optimal power conversion efficiencies obtained with CSDPP5, CSDPP6, CSDPP7 and CSDPP8 are 2.97% 3.06%, 2.42% and 2.43% respectively. The PCE of the devices when processed with DIO/THF solvent, have been further enhanced to 4.69%, 4.14% for CSDPP6:PC71BM and CSDPP8:PC71BM active layers respectively. The enhancement in PCE has been attributed to change in nanoscale morphology and more balanced charge transport resulting from increased hole mobility.

 Please wait while we load your content...
Please wait while we load your content...