Basicity and stability of urea deep eutectic mixtures†
Abstract
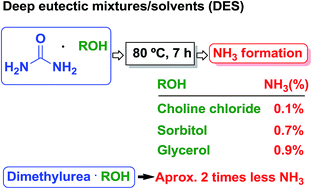
The stability and basicity origin of some common urea based deep eutectic mixtures/solvents (DES) were studied. We have observed an unexpected Hantzsch dihydropyridine reaction in sorbitol/urea DES, where the source of ammonia was found not to be originated from the individual ingredients of the DES. Our results showed that decomposition of urea occurs in DES at lower than expected temperatures, namely below 100 °C and is enhanced in diol DES by the formation of carbonates causing their unexplained basicity. Carbohydrates and choline chloride (ChCl) DES exhibit lower rates of decomposition, while no decomposition was observed from neat urea or MeOH and EtOH urea solutions.

 Please wait while we load your content...
Please wait while we load your content...