Phosphorhydrazide inhibitors: toxicological profile and antimicrobial evaluation assay, molecular modeling and QSAR study†
Abstract
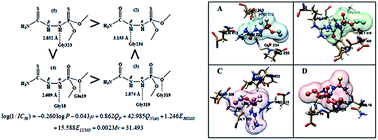
A series of phosphorhydrazide (PHA) derivatives with the (X = O,S) P–NHα–NHβ–C (X = O,S) skeleton (1–23) were synthesized and characterized by spectral techniques. A single crystal X-ray study of 4 and 21 provided confirmation of the hydrogen bonding structures. The synthesized compounds exhibited drastically reduced antibacterial activity against Gram-positive and -negative bacteria compared to the reference drugs. The insecticide activity of the PHAs appraised for the elm leaf beetle demonstrated that (CH3O)2(S)P–NHα–NHβ–C(O)(C4H4O) has more effect than the other compounds in inhibiting α-esterase. Docking analysis showed that hydrogen bonds were formed between the N–Hα protons of the (S)P–NHα–NHβ–C(S), (O)P–NHα–NHβ–C(S) and (O)P–NHα–NHβ–C(O) moieties with Gly323, Gly18 and Gly319 as well as the N–Hβ proton of the (S)P–NHα–NHβ–C(O) moiety and the AChE receptor site (Gly234). According to the QSAR model, the net charge of the N–Hα (QN(α)) nitrogen atom contributes an important electronic function in the inhibition of AChE. A high interrelationship between QN(α) and QP proved that the NH–P(X) moiety has a higher inhibitory activity than the NH–C(X) moiety.

 Please wait while we load your content...
Please wait while we load your content...