Selective synthesis of Fe3O4, γ-Fe2O3, and α-Fe2O3 using cellulose-based composites as precursors
Abstract
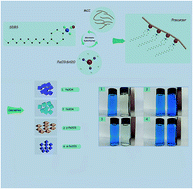
Iron oxide with various phases such as Fe3O4, γ-Fe2O3, and α-Fe2O3 has been selective successfully synthesized using cellulose-based composites as precursors, which were obtained at 180 °C for 45 min by the microwave-hydrothermal method. The products were characterized with X-ray powder diffraction (XRD), Fourier transform infrared spectroscopy (FT-IR), thermogravimetric analysis (TG), derivative thermogravimetric analysis (DTG), X-ray photoelectron spectroscopy (XPS), and scanning electron microscopy (SEM). Fe3O4, γ-Fe2O3, and α-Fe2O3 could be selectively synthesized by changing the calcination temperature and atmosphere. It was found that Fe3O4 was obtained in the N2 atmosphere. However, γ-Fe2O3 and α-Fe2O3 were observed at 300 and 500 °C for 3 h in the air atmosphere, respectively. The as-synthesized Fe3O4 and γ-Fe2O3 displayed superparamagnetic characteristics at room temperature via the magnetization measurement. The methylene blue (MB) adsorption capacity of γ-Fe2O3 (air 300 °C) can reach 98.72% and the adsorption isotherms better fitted Freundlich isotherm models. This work provided a promising strategy for the synthesis of metal and carbon/metal composites using biomass as a precursor.

 Please wait while we load your content...
Please wait while we load your content...