One-pot C–C/C–O bond formation: synthesis of spirocyclic lactones†
Abstract
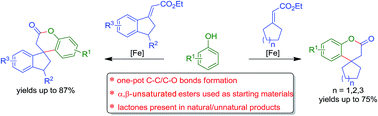
An efficient and practical method for the synthesis of novel spiro(tri or tetra)cyclic lactones via the formation of C–C and C–O bonds in one-pot is presented. The method was successful under Lewis acidic (FeCl3) conditions and enabled the formation of spiro(tri or tetra)cyclic lactones from simple aliphatic exocyclic enoate esters as reacting partners with phenols. Remarkably, the usual self-aromatization of cyclohexanone based enoate esters, under such Lewis acidic conditions is overridden by intermolecular coupling. Significantly, the method was amenable to indanone derived esters as well and furnished novel spiro(tetra or penta)cyclic lactones bearing simple to dense functionalities on the aromatic rings. Notably, these novel spirocyclic systems constitute core structures of natural/unnatural compounds that show good biological properties.

 Please wait while we load your content...
Please wait while we load your content...